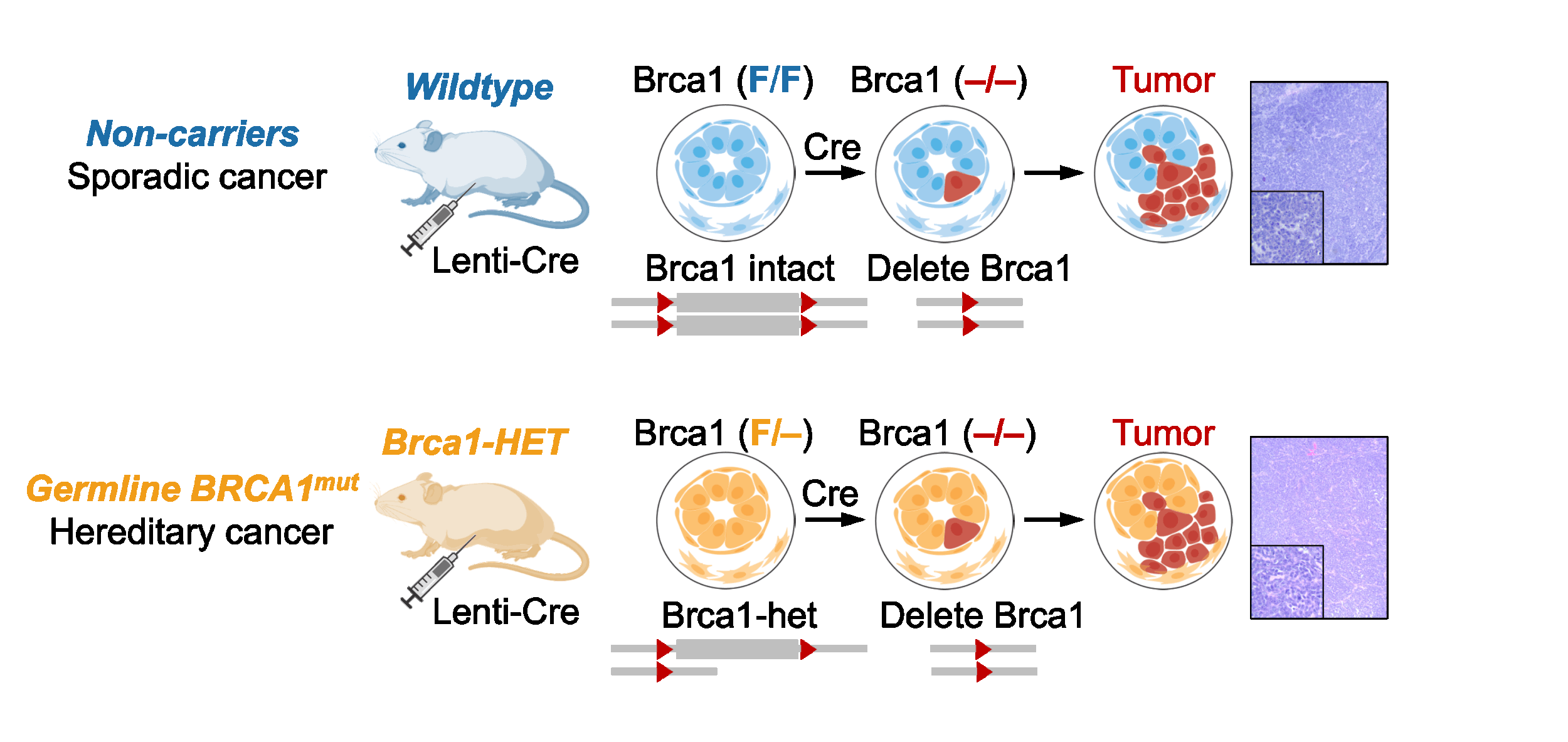
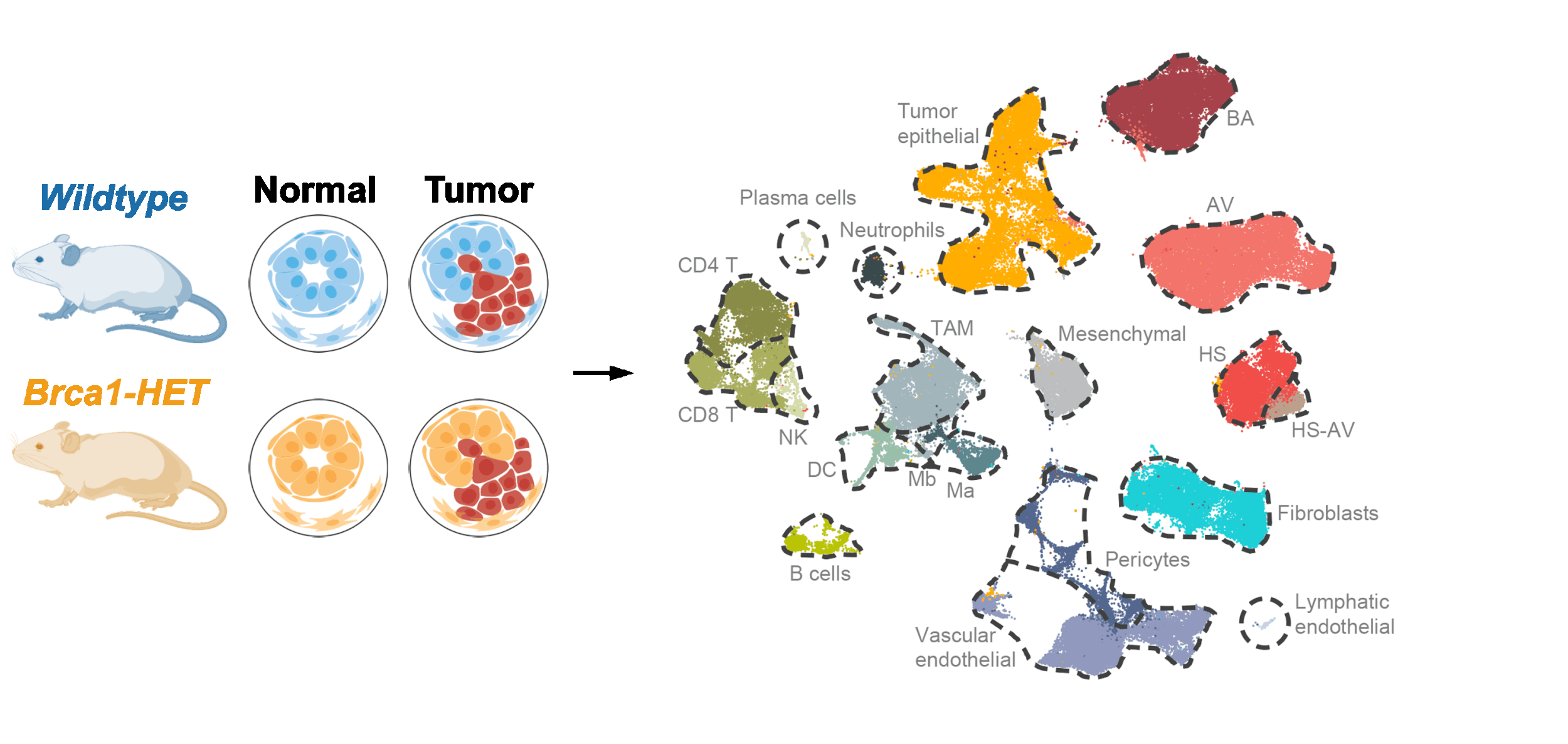
Mechanisms of hereditary cancer development
Why do people with certain inherited mutations have higher cancer risks, even at a young age? These “hereditary cancers”, driven by heterozygous loss-of-function mutations in tumor suppressor genes, impact multiple generations, and better prevention strategies are sorely needed. Emerging evidence from us and others indicates that this cancer predisposition cannot be fully explained by the prevailing “two-hit” hypothesis (i.e., carrying one instead of two copies of a protective gene simply increases a cell’s risk of completely losing the gene to suppress malignant transformation). Rather, the heterozygous state itself can cause cellular and molecular abnormalities that substantially elevate cancer risk, independent of full gene loss.
Our lab investigates the causes and consequences of these heterozygosity-driven gene-haploinsufficiency effects on hereditary cancer development and explores opportunities for early disease intervention. We combine genetically engineered mouse models, organoid cultures, and advanced omics analyses to study hereditary cancers in a tractable and physiological manner. Our long-term goal is to build a unifying paradigm of hereditary cancers, with shared principles and disease-specific mechanisms. Insights from these studies can also shed light on non-hereditary cancers.
Key questions
1. Molecular mechanisms
What alterations are induced by germline heterozygous mutations? How do they arise and what are their effects on tumorigenesis?
2. Risk factors
How do these changes intersect with physiological factors (e.g., pregnancy, obesity, and aging) to impact cancer risk?
3. Therapeutic opportunities
Can we leverage these aberrations to discover biomarkers and targets for early cancer intervention?
Approaches
Genetically engineered mouse models of cancer
Organoid cultures
Single-cell and bulk omics analyses